Original article (in Albanian) was published on 8/5/2024; Author: Pustina Patris
This new declaration was made in a British court, although the company was aware of the risk of clotting.
Claim: AstraZeneca has admitted for the first time that its anti-Covid vaccine can cause side effects
Verdict: Missing context
—————————————–
By late April 2024, several Albanian-language portals and media outlets had reported that the pharmaceutical company had admitted, ‘for the first time’, that its Covid vaccines could cause side effects, specifically blood clots. The following are some of the headlines.
| AstraZeneca has admitted for the first time that its anti-Covid vaccines have caused serious side effects following complaints from victims Published on 29.04.2024 by shekulli_admin |
| AstraZeneca admits for the first time: The Covid-19 vaccine causes blood clots |
| AstraZeneca admits for the first time that its vaccines have caused serious side effects, following complaints from victims (photo) |
| AstraZeneca admits for the first time that its Covid-19 vaccine may cause side effects |
The articles refer to a collective lawsuit in the UK against the pharmaceutical company, claiming that the Vaxveria vaccine caused serious illness and death in dozens of cases.
According to the British newspaper The Telegraph, in a letter sent to the lawyers of one of the claimants, Jamie Scott, in May 2023, AstraZeneca stated ‘We do not accept that thrombosis with thrombocytopenia syndrome (TTS) is caused by the vaccine at a general level’. However, in a legal document filed with the UK High Court in February 2024, AstraZeneca stated: ‘It is accepted that the AZ vaccine may, in very rare cases, cause TTS. The causative mechanism is not known. Furthermore, TTS can occur even in the absence of the AZ vaccine (or any vaccine). Causation in each individual case will be a matter for expert evidence’.
Thus, it appears that AstraZeneca denied a causal link between the vaccine and TTS in communication with the lawyers of a claimant, but when this denial would constitute a legal statement, the company admitted a possible link in court.
However, this is not the first time that AstraZeneca has acknowledged this potential risk of the vaccine.
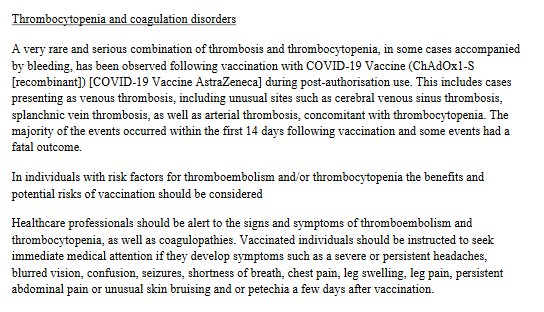
In a product information leaflet accompanying the AstraZeneca vaccine since April 2021, it is stated that ‘a very rare and serious combination of thrombosis and thrombocytopenia, in some cases with bleeding, has been observed following vaccination with the COVID-19 Vaccine (ChAdOx1-S [recombinant]) [AstraZeneca CoVID-19 vaccine] during the application following the authorization’.
Furthermore, on March 18, 2021, the European Medicines Agency (EMA), after reviewing reports of blood clots in individuals vaccinated with AstraZeneca, announced that ‘the vaccine may be associated with very rare cases of blood clots linked with thrombocytopenia’.
In September 2021, AstraZeneca issued a press releaseacknowledging that ‘cases of thrombosis with thrombocytopenia (TTS) have been reported in a small number of people. Early diagnosis allows appropriate treatment for these cases, and there is no increased risk of TTS in the second dose compared to the levels expected in the general population’.
Thus, the existence of this rare side effect has been public information since 2021 and has been acknowledged by health agencies and AstraZeneca itself at that time.